Disinfectants play vital roles in biosecurity and infection control across various sectors, including human health, laboratory, veterinary, and laboratory settings. Depending upon their application, disinfectants are required to be registered as medical devices or biocides. While they may share similar composition and efficacy profiles, medical devices and biocidal disinfectants are regulated differently in the UK and EU, and it can be difficult to know which is required for a particular purpose. In this blog post, our Senior Regulatory Manager, Anna Williams, simplifies the regulations and explains when to use each type of disinfectant based on specific industries.
Disinfectants in Human Healthcare Environments
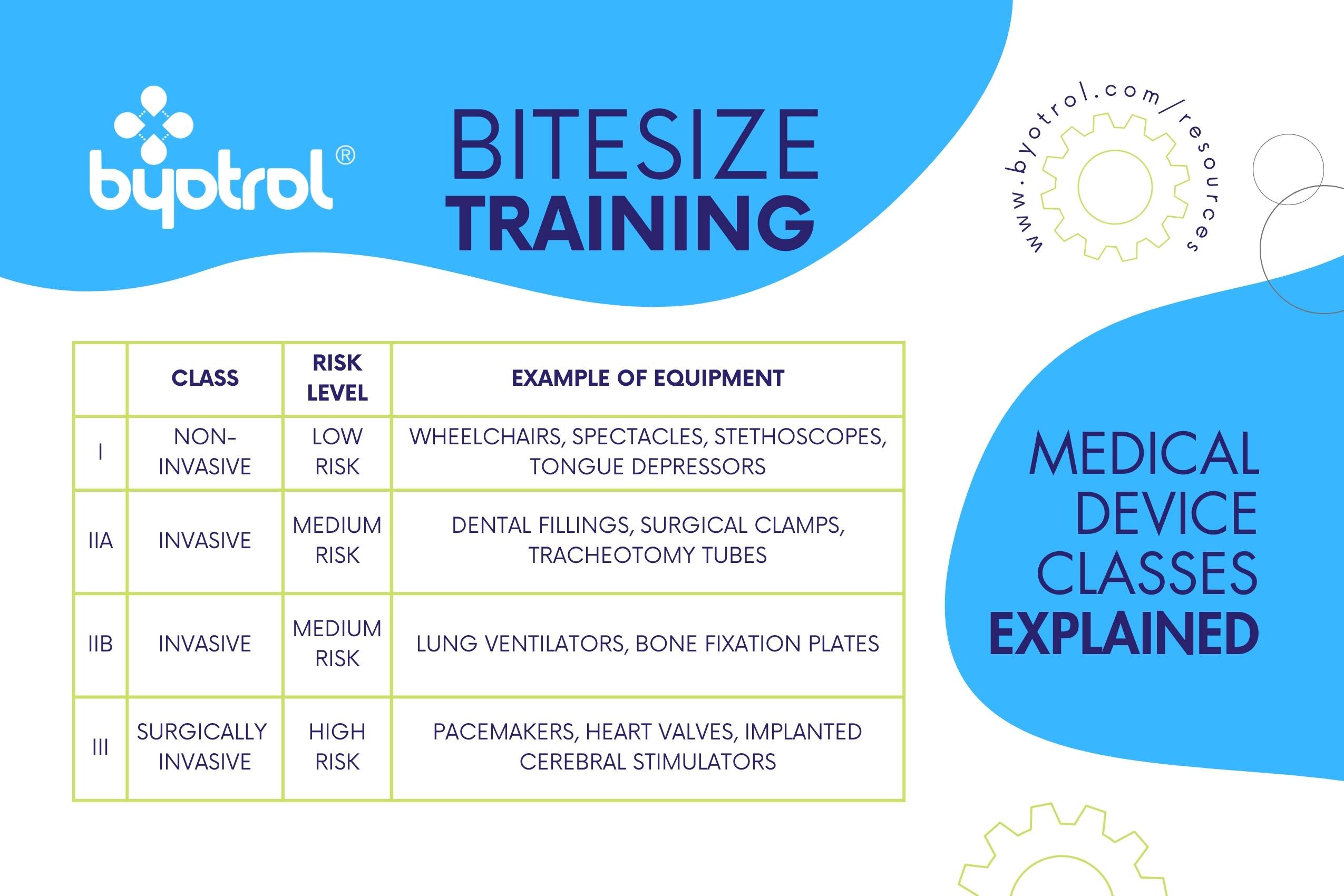
In the medical industry and human healthcare environments, it’s crucial to use the right disinfectant for different devices. CE-marked medical devices, like stethoscopes, wheelchairs, and pacemakers, require a CE-marked medical device disinfectant. It’s important to ensure the disinfectant is approved for the specific Class of device. For example, a medical device disinfectant designed and approved for disinfecting Class I non-invasive medical devices (e.g., stethoscopes) cannot be used to disinfect Class IIa invasive (e.g., dental fillings) or Class III surgically invasive (e.g., pacemakers) medical devices. This should be clear on the product label, but always consult your supplier if you are unsure.
Which Class of Medical Device Do I Need?
For non-medical device surfaces and environmental cleaning and disinfection, such as countertops and floors, a biocidal disinfectant is appropriate. Similarly, equipment and surfaces within morgues or mortuaries can also be disinfected with biocidal products, not medical devices. An individual disinfectant can be registered as both a medical device disinfectant and a biocidal disinfectant, but the regulations differ and are often governed by different regulatory bodies, therefore you should consult your supplier to gather evidence of registration under both regulations to avoid accidental non-compliance.
What About the Care Industry?
Care homes focus on patient welfare, often utilising medical devices like defibrillators and patient monitors. Like in a hospital, CE-marked medical device disinfectant should be used for these devices, adhering to the appropriate Class of medical device. For areas like food-preparation spaces, communal areas, and bathrooms, you must use a biocidal disinfectant.
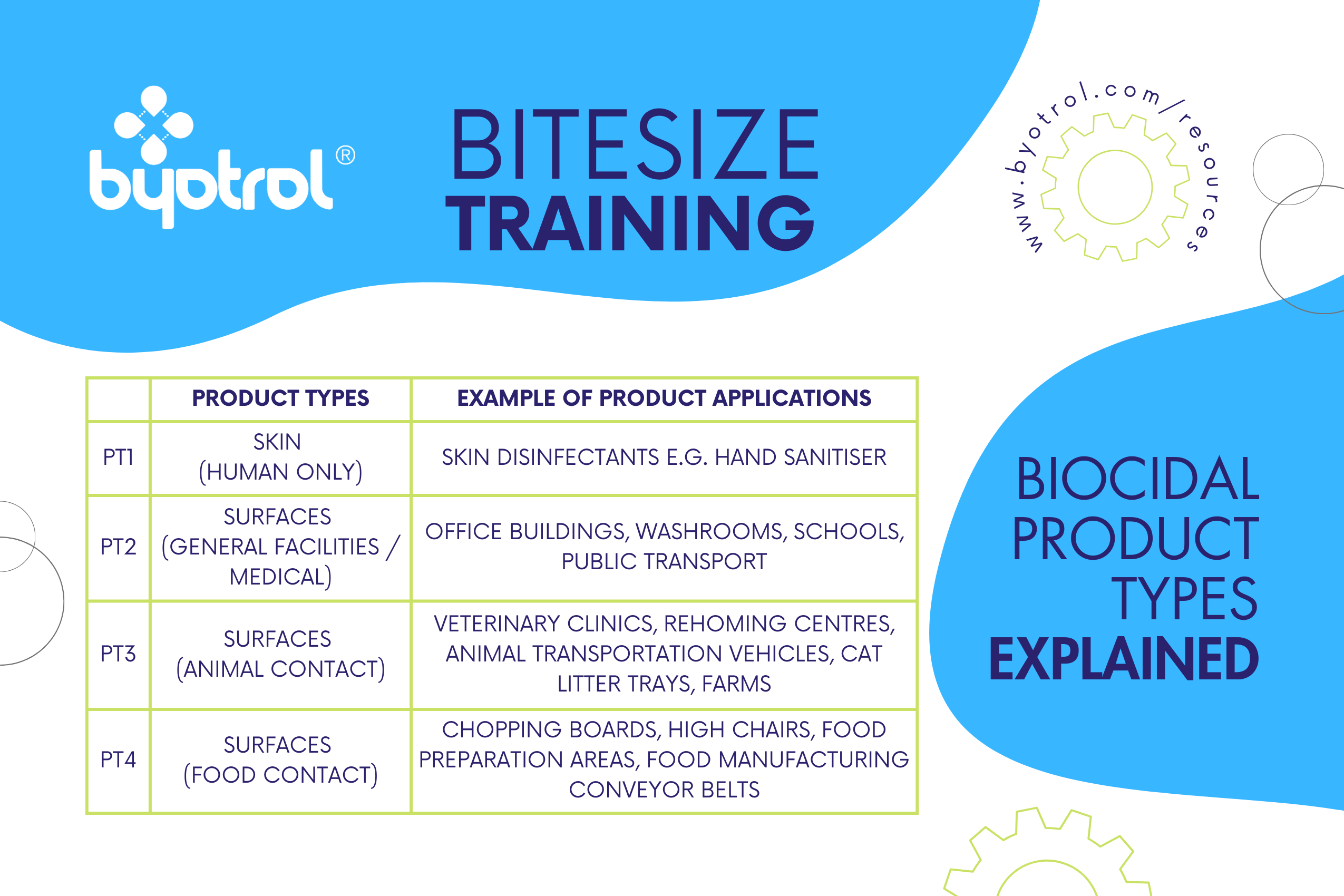
How Do I Know Which Biocidal Product Type is Correct?
Like medical devices, biocidal products may only be approved for specific areas and are split by product type (PT). You should verify that the chosen biocidal disinfectant is approved for the intended area for use. A disinfectant approved for communal areas or bathrooms would need to use active substances approved for PT2 and must be tested to PT2 protocols, however, disinfectants used in food-preparation areas should adhere to the requirements for PT4.
Are Medical Device Disinfectants Required in Veterinary Environments?
In veterinary settings or organisations that transport, care for and house animals, biocidal disinfectants should always be used. The scope of the Medical Device Regulation focuses solely on the intended use “for human beings”, therefore disinfectants used in facilities for the care and treatment of animals should be biocidal. Care should be taken to select a biocidal disinfectant approved for PT3 as some disinfectants may not be effective against pathogens found in animal environments.
For more information on the requirements for a PT3 biocidal disinfectant please read our previous blog post, which goes into greater depth on the regulations for veterinary disinfectants and provides helpful questions to ask your disinfectant manufacturer.
What Type of Disinfectant is Appropriate for Laboratories?
Laboratories may require both medical device and biocidal disinfectants, depending on the instruments and surfaces involved. Instruments not designed for human contact, like pipettes should be disinfected with a biocidal disinfectant. Only medical devices necessitate a medical device disinfectant. Additionally, other surfaces in the laboratory, such as lab benches and ultrasonic baths should be cleaned and then disinfected using a biocidal disinfectant.
But Why Does It Matter?
Unfortunately, just making sure your disinfectant is effective isn’t enough for legal compliance. Using the correct type of disinfectant is extremely important, as your organisation is not legally compliant if the wrong type is used. Medical device disinfectants are tailored for specific devices, while biocidal disinfectants cover a broader range of surfaces. Understanding the distinction between the two ensures effective biosecurity and infection control, and maintains the compliance of your organisation, and your team.
For further enquiries or additional industry-specific information, feel free to contact us.